Developing Next-generation Treatments to Fight Neurodegenerative and Other Misfolded Protein Diseases
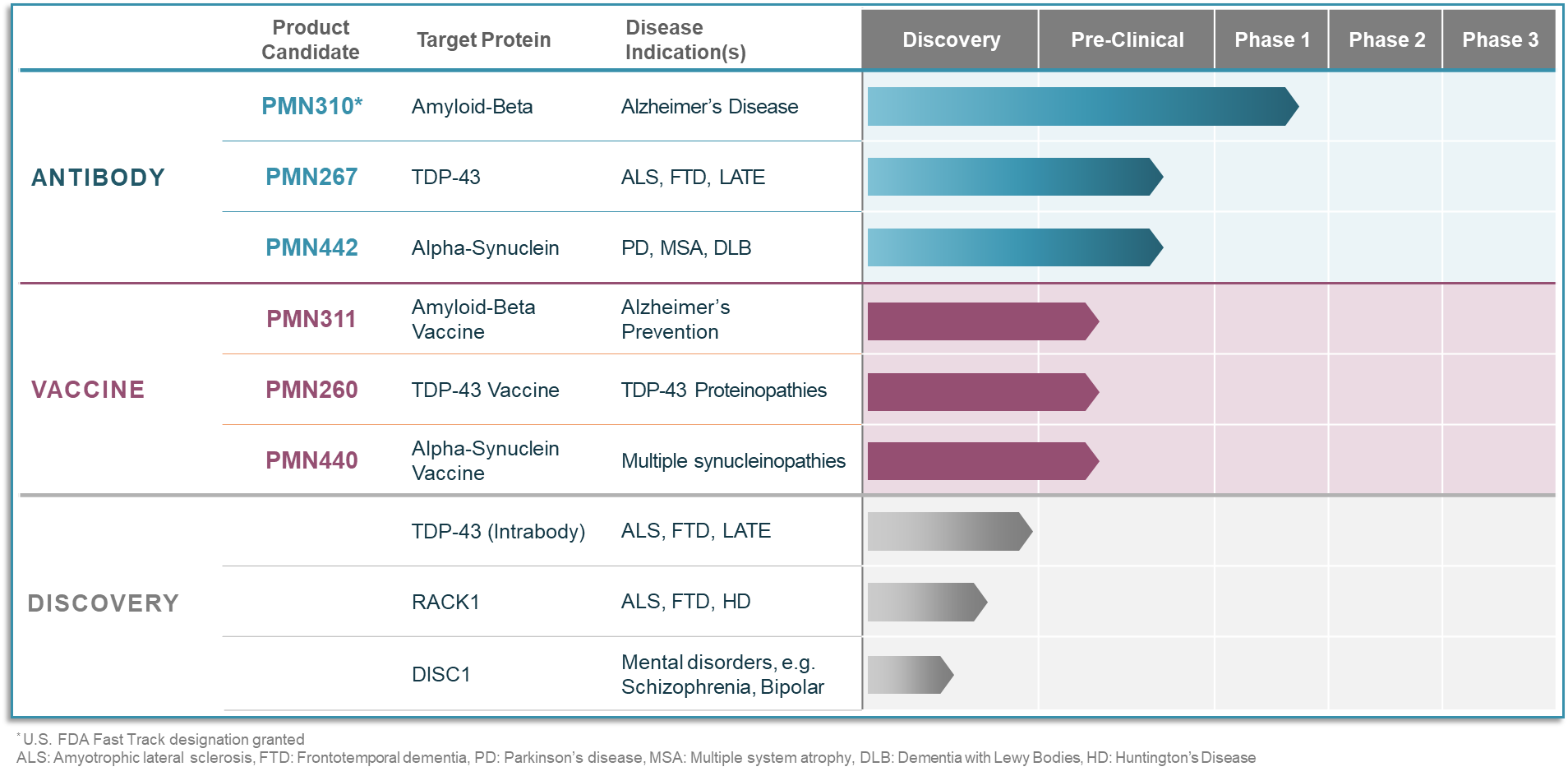
At ProMIS Neurosciences, we use state-of-the-art, proprietary computational modeling to predict and identify specific targets (epitopes) expressed on the molecular surface of toxic oligomers (misfolded proteins), which are known root causes of several neurodegenerative and other misfolded protein diseases. These oligomers are toxic to neurons and can spread throughout the brain or spinal cord, killing neurons, and leading to the development and progression of neurodegenerative and other misfolded protein diseases.